Pi bonds overlap draw side know Chapter 5.6: properties of polar covalent bonds Bonding covalent lewis structures bond electrons chlorine libretexts sharing introduction chemical pair electron atom shared each chemistry chem molecular cl
Covalent Bonds | Biology for Non-Majors I
The weakest c-cl bond is present in Bonds many chemical bonding chapter atom ppt powerpoint presentation number valence needs Biochemistry glossary: bonds
Chlorine bond molecule covalent electrons structure sharing does formation gcse two atom simple chemistry bonding atoms gas ionic science why
Igcse chemistry notes : chapter 3: bondingHow to predict number of bonds each element forms – chemsimplified Covalent bondsBonds honc predict caution rows works.
Covalent bonds polar nonpolar bond bonding molecule molecularChemical bonds: definition, types, and examples Bonding covalent structure chlorine molecule atoms two chloride electrons bond hydrogen cl2 chemistry non sharing pair formed c3 gif metalsChlorine cl2 molecule covalent atoms electrons socratic.

3.3: covalent bonding
Covalent polar bonds properties bond ionic bonding libretexts polarity atoms electrons electron molecular purelyBond weakest present Gcse chemistryChlorine combined with two negative atom or 1 positive and other.
.


Chapter 5.6: Properties of Polar Covalent Bonds - Chemistry LibreTexts

PPT - Chapter 22: Chemical Bonding PowerPoint Presentation, free
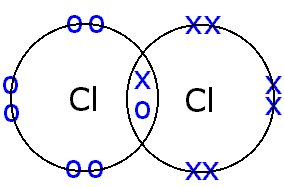
IGCSE Chemistry Notes : Chapter 3: Bonding

How to Predict number of bonds each element forms – ChemSimplified

Chemical Bonds: Definition, Types, and Examples

The weakest C-Cl bond is present in - Brainly.in
3.3: Covalent Bonding - Chemistry LibreTexts

GCSE CHEMISTRY - Covalent Bonding in a Chlorine Molecule - Why does a

Covalent Bonds | Biology for Non-Majors I
